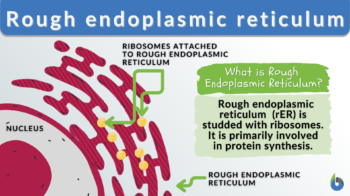
Rough endoplasmic reticulum
n., plural: rough endoplasmic reticula
[ɹʌf ˈɛndəʊˌplăz′mɪk rɪˈtɪkjʊləm]
Definition: A type of endoplasmic reticulum wherein polyribosomes are attached to the surfaces of the endoplasmic reticulum membrane
Table of Contents
Rough Endoplasmic Reticulum Definition
The rough endoplasmic reticulum (rough ER or rER) is a membrane-bound organelle found in eukaryotic cells. It is characterized by its rough appearance in electron micrographs due to the presence of ribosomes attached to its membrane. The rough ER is primarily involved in various cellular processes, such as protein synthesis, protein folding, post-translational modifications, and quality control.
The ribosomes on the surface of the rough ER synthesize proteins based on the instructions encoded in messenger RNA (mRNA). As the nascent polypeptide chains are synthesized, they enter the lumen of the rough ER, where they undergo folding and modifications (such as glycosylation and disulfide bond formation). These modifications are crucial for the proper structure and function of proteins.
The rough ER plays a vital role in the quality control of proteins. It houses chaperone proteins that assist in proper protein folding, as well as quality control mechanisms to identify misfolded or aberrant proteins. Misfolded proteins are targeted for degradation through the ER-associated degradation (ERAD) pathway to maintain cellular protein homeostasis.
In addition to its role in protein synthesis and folding, the rough ER is involved in membrane biogenesis. It contributes phospholipids and membrane proteins to the expanding endomembrane system, including other organelles such as the Golgi complex and plasma membrane.
The rough endoplasmic reticulum is an essential organelle for cellular function and is particularly abundant in cells with high protein synthesis demands, such as secretory cells and neurons. Dysfunction of the RER and protein misfolding within it have been implicated in various diseases, highlighting its importance in maintaining cellular homeostasis.
The rough endoplasmic reticulum is a type of (or a region in the) endoplasmic reticulum characterized by its rough appearance due to the ribosomes attached to its membrane. Because of the ribosomes associated with it, the rough endoplasmic reticulum is primarily concerned with protein synthesis and quality control processes to maintain cellular protein homeostasis.
Synonym: granular endoplasmic reticulum
Abbreviation: rER; rough ER.
Rough Endoplasmic Reticulum Overview
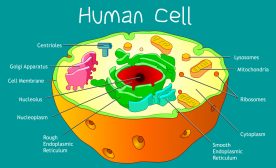
The rough ER is one of the two major regions of the endoplasmic reticulum; the other is the smooth endoplasmic reticulum. Sarcoplasmic reticulum is also a type of ER but it is specifically a subtype of the smooth ER present in muscle cells. They are distinguished by their distinctive morphology and functions. Here is the Table summarizing the differences between the rough and the smooth endoplasmic reticulum. Both these regions are characterized by their involvement in the synthesis and metabolism of macromolecules, particularly, proteins and lipids. The ER, on the whole, is a membrane-bound organelle in eukaryotic cells (i.e., protists, fungi, plant cells, and animal cells). It consists of an interconnected network of flattened sacs, tubules, and vesicles. It is continuous with the outer membrane of the nuclear membrane (nuclear envelope) that spans the cytoplasm and may extend to the cell membrane.
Watch this vid to learn the differences between smooth and rough ER:
Rough Endoplasmic Reticulum Discovery
The discovery of the rough endoplasmic reticulum (RER) may be credited to various scientists. In the 1940s, Keith R. Porter, a cell biologist, named the cellular structure consisting of a network of flattened sacs and tubules “endoplasmic reticulum” based on its appearance. Then, in the early 1950s, George E. Palade, another cell biologist, coined the term “rough endoplasmic reticulum” to refer to the region of the endoplasmic reticulum studded with ribosomes. Soon, the functions of the rough endoplasmic reticulum have been elucidated, particularly its role in protein synthesis, post-translational modifications, and the secretory pathway, including its involvement in certain pathobiologies.
Rough Endoplasmic Reticulum Structure
How do you identify the rough endoplasmic reticulum from other organelles, including the smooth endoplasmic reticulum? Let’s find out!
- Single lipid bilayer membrane. The rough endoplasmic reticulum, like the rest of the ER, has a single lipid bilayer membrane.
- Flattened sacs. It is the region in the ER consisting of flattened sacs called cisternae.
- Rough ER lumen. The contents of the rough endoplasmic reticulum lumen are chiefly enzymes and chaperone proteins for protein folding, modifications, and initial stages of transport.
- Dynamic structure. It is a rather dynamic organelle that undergoes constant remodeling and fusion with other cellular compartments. Its structure may vary depending on the cell type and its function. For instance, it may form elaborate networks with extensive branching especially when there is a marked increase in the demand for certain proteins. At other times, it may be less prominent, when the cell is involved in other cellular activities more than protein synthesis.
- Interconnected, for intracellular communication. The rough ER lumen is interconnected with the other regions of the ER, (e.g., transitional ER and smooth ER). Thus, exchanges of proteins, lipids, calcium ions, and other molecules can occur via the ER lumen. The rough ER’s protein products, for instance, can flow across the ER lumen, and be transported via membrane contact sites with the other organelles, or shuttled as “cargoes” within transport vesicles that bud off from the transitional ER.
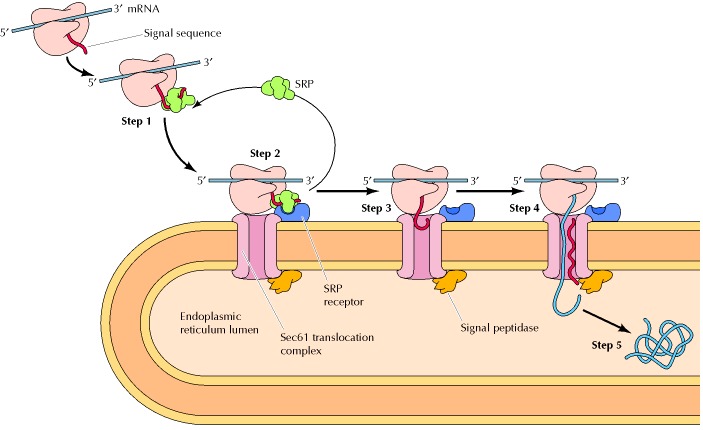
- Rough ER markers. One of the markers of the rough ER is the presence of translocon, i.e. a multiprotein complex involved in the translocation of nascent polypeptides from the cytosol to the interior (lumen) of the rough ER across the rough ER membranes. (see Figure 1) The translocon serves as the binding site where ribosomes can dock to the endoplasmic reticulum. The bound ribosomes, though, are attached to the ER transiently. They may come and go. They attach to the endoplasmic reticulum (via the translocon) when a signal peptide is synthesized (i.e. by protein translation at the ribosome) and then recognized by a signal recognition particle (SRP). Another typical rough ER marker is ribophorin I, which is a transmembrane protein in mammalian cells that facilitates the proper positioning and orientation during the translocation of the nascent polypeptide into the lumen of the rough ER.
Overall, the structure of the rough ER is specialized to support its primary function in protein synthesis and processing. Its interconnected network allows for efficient production, folding, and modification of proteins, contributing to cellular functions and maintaining protein homeostasis.
Rough Endoplasmic Reticulum Functions
The rough ER is primarily involved in protein synthesis and in monitoring proper protein folding so that it can be shuttled to its next destination, such as the Golgi apparatus where the nascent protein can further undergo maturation and be labeled for its final destination.
While smooth ER is better known as the region in the ER that synthesizes lipids, rough ER has also been found to make lipids to a certain extent. The rough ER is also involved in the manufacture of lysosomal enzymes (in which a marker, mannose-6-phosphate, is later added in the Golgi apparatus). It is also where certain integral membrane proteins are formed. N-linked glycosylation also occurs here (O-glycosylation occurs in the Golgi).
-
Protein synthesis, folding, and transport
Protein synthesis is the process of creating protein molecules. The major steps of protein synthesis are (1) amino acid synthesis (amino acids are produced for use during mRNA transcript translation), (2) transcription (mRNA copies the protein code from the DNA), and (3) translation (the transcript is translated into amino acids that are joined in their proper sequence). This is followed by a series of maturation events, such as proteolysis, protein folding, and other post-translational modifications.
For the steps, read: Protein Synthesis and Folding
-
ER stress response
The build-up of unfolded or misfolded protein triggers a cellular response wherein the improperly folded proteins are taken out of the lumen and then brought to the cytosol for degradation. This is referred to as the endoplasmic reticulum stress response or the unfolded protein response (UPR response).
In summary, the rough ER plays a pivotal role in the UPR by facilitating protein folding and quality control, recognizing and retro-translocating misfolded proteins, activating UPR signaling pathways, and participating in ERAD. These functions collectively contribute to maintaining ER homeostasis and ensuring the proper folding and clearance of proteins to alleviate ER stress.
In response to the accumulating misfolded or unfolded proteins in the ER lumen, the rough ER takes the following measures:
-
- Protein Folding and Quality Control: Molecular chaperones and folding enzymes in the rough ER facilitate the correct folding of proteins. During ER stress, when misfolded proteins accumulate, the rough ER responds by upregulating the expression of chaperones and folding enzymes. This will enhance protein folding capacity and promote the quality control of newly synthesized proteins.
- Recognition and Retrotranslocation of Misfolded Proteins: The rough ER has an inherent quality control mechanism to recognize misfolded proteins. If a protein ended up with a conformation that is improper or dysfunctional, the ER-resident chaperones and quality control sensors, such as calnexin, calreticulin, and binding immunoglobulin protein (BiP) will recognize it. The sensors will interact with the misfolded protein and facilitate its retrotranslocation or extraction from the ER lumen back to the cytosol for degradation.
- Activation of UPR Signaling Pathways: The accumulation of misfolded proteins in the rough ER triggers the activation of UPR signaling pathways. The UPR sensors present in the ER membrane, such as inositol-requiring enzyme 1 (IRE1) and protein kinase RNA-like ER kinase (PERK), detect the presence of unfolded proteins and initiate signaling cascades that will counter ER stress. These pathways involve the activation of transcription factors, such as X-box binding protein 1 (XBP1) and activating transcription factor 4 (ATF4), which regulate the expression of genes involved in protein folding, ER-associated degradation (ERAD), and other UPR-related processes.
- ER-Associated Degradation (ERAD): The rough ER, together with the cytoplasmic proteasome, participates in ER–associated degradation (ERAD) of misfolded proteins. ERAD involves the retrotranslocation of misfolded proteins from the ER lumen or membrane into the cytosol, where they are ubiquitinated and targeted for proteasomal degradation.
Rough Endoplasmic Reticulum Locations
Cell types that have prominent rough ER and are primarily active in protein synthesis are as follows:
- Mucus-secreting cells, such as the cells of the respiratory and the gastrointestinal systems
- Plasma cells that produce antibodies
- Exocrine cells that produce and secrete digestive enzymes
- Endocrine cells that produce protein hormones (such as insulin)
- Hepatocytes (liver cells) that produce plasma proteins, such as albumin and clotting factors
- Cells of the adrenal cortex that synthesize steroid hormones (e.g., cortisol and aldosterone)
Disorders Of Dysfunctional Rough Endoplasmic Reticulum
Dysfunction of the rough endoplasmic reticulum (ER) can contribute to various medical conditions, particularly those involving protein folding and trafficking. Here are a few examples:
-
Protein misfolding disorders
Mutations or defects in proteins that are synthesized and folded in the rough ER can lead to protein misfolding disorders. These include diseases like peripheral insulin resistance, cystic fibrosis, alpha-1 antitrypsin deficiency, and certain forms of neurodegenerative disorders like Alzheimer’s disease and Parkinson’s disease.
-
Congenital disorders of glycosylation
Congenital disorders of glycosylation are caused by defects in the biosynthesis as well as in the processing of protein-bound glycans in the rough ER. Some of the symptoms include developmental delays, intellectual disability, skeletal abnormalities, and organ dysfunction.
-
Wolcott-Rallison Syndrome
This is a rare genetic disorder characterized by early-onset diabetes, skeletal dysplasia, and liver dysfunction. The underlying cause is mutations in the EIF2AK3 gene, which could end up in improper regulation of protein synthesis and ER stress response.
-
ER storage diseases
The accumulation of lipid molecules within the rough ER could lead to certain metabolic disorders, such as Gaucher disease and Niemann-Pick disease. In particular, these conditions could lead to organ dysfunction, neurodegeneration, and other systemic symptoms.
-
Retinitis pigmentosa
Retinitis pigmentosa is an inherited eye disorder causing progressive vision loss. One of the causes is mutations in the genes that code for proteins involved in protein synthesis and folding in the rough ER. The dysfunctional gene thus impairs the function of the retinal cell and ultimately causes the death of the retinal cell.
Rough ER dysfunction can be one component of these conditions, and their pathogenesis is often complex as it involves multiple cellular processes and organelles. Each disorder has its own specific mechanisms and manifestations related to rough ER dysfunction.
NOTE IT!
“Rough Endoplasmic Reticulum Takeaways”
Here are some interesting facts about the rough endoplasmic reticulum (rough ER):
- Name origin: The term “rough” in rough endoplasmic reticulum refers to the presence of membrane-bound ribosomes on its surface, giving it a rough appearance in electron micrographs.
- Protein synthesis: The primary function of the rough ER is to synthesize proteins. It has membrane receptors that will interact with the ribosome-mRNA-signal peptide complex. Once docked, the translation of mRNA resumes, producing a polypeptide chain that is translocated into the ER lumen for further processing.
- Folding and modification: The rough ER provides an environment for proper protein folding and post-translational modifications, such as glycosylation and disulfide bond formation. These modifications are crucial for protein stability and functionality.
- Quality control: The rough ER plays a vital role in protein quality control. It houses chaperone proteins that monitor protein integrity. The rough ER has its machinery that recognizes misfolded or aberrant proteins and then removes them from the lumen for degradation (via the ER-associated degradation, ERAD, pathway).
- Membrane biogenesis: The rough ER is involved in the synthesis of new membrane components. It contributes phospholipids and membrane proteins to the expanding endomembrane system, including other organelles such as the Golgi apparatus and plasma membrane.
- Lipid metabolism: While the rough ER is primarily known for its role in protein synthesis, it is also involved in lipid metabolism. It participates in the biosynthesis of phospholipids, cholesterol, and other lipids, which are essential components of cellular membranes.
- Secretory pathway: Proteins synthesized in the rough ER are often destined for secretion or membrane insertion. Secretory proteins are transported from the rough ER to the Golgi apparatus and subsequently to their final cellular destinations through the secretory pathway.
- Role in disease: Dysfunction of the rough ER and protein misfolding within it are associated with various diseases, including neurodegenerative disorders such as Alzheimer’s and Parkinson’s, as well as certain genetic disorders like cystic fibrosis.
- Neuronal function: The rough ER is particularly abundant in neurons, where it plays a critical role in the synthesis and transport of membrane proteins required for neuronal function and communication.
- Evolutionary conservation: The rough endoplasmic reticulum is a highly conserved organelle in eukaryotic cells. It is present in a wide range of organisms, from simple single-celled organisms to complex multicellular organisms.
These facts highlight the diverse and essential functions of the rough endoplasmic reticulum in cellular processes, protein synthesis, and overall cell homeostasis.
Take the Rough endoplasmic reticulum – Biology Quiz!
References
- Palade, G. E. (1956). Intracellular aspects of the process of protein synthesis. Science, 124(3215), 77-83.
- Novick, P., & Schekman, R. (1979). Secretion and cell-surface growth are blocked in a temperature-sensitive mutant of Saccharomyces cerevisiae. Proceedings of the National Academy of Sciences, 76(4), 1858-1862.
- Nicchitta, C. V. (2002). Biochemical, cell biological, and immunological issues surrounding the endoplasmic reticulum chaperone GRP94/gp96. Current Opinion in Immunology, 14(1), 19-26.
- Aridor, M., & Balch, W. E. (2000). Principles of selective transport: coat complexes hold the key. Trends in Cell Biology, 10(5), 203-210.
- Wang, M., & Kaufman, R. J. (2016). Protein misfolding in the endoplasmic reticulum as a conduit to human disease. Nature, 529(7586), 326-335.
- Schroder, M., & Kaufman, R. J. (2005). ER stress and the unfolded protein response. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis, 569(1-2), 29-63.
- Walter, P., & Ron, D. (2011). The unfolded protein response: from stress pathway to homeostatic regulation. Science, 334(6059), 1081-1086.
- Zhang, K., & Kaufman, R. J. (2008). From endoplasmic-reticulum stress to the inflammatory response. Nature, 454(7203), 455-462.
©BiologyOnline.com. Content provided and moderated by Biology Online Editors.